RNS-320 Patients Now Have Access to Valuable Imaging Options
FULL BODY MRI CONDITIONAL
ALL RNS-320 PATIENTS
Current and future, can receive MRIs
NO RESTRICTIONS ON LEADS
THE RNS® SYSTEM CAN BE IMPLANTED BEFORE LASER ABLATION*
MRI Mode on the RNS System
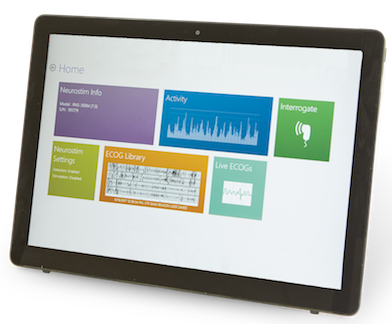
Peace of Mind
Check on your patients any time to see the status of MRI Mode using the Patient Data Management System
Easy to Use
Easily activate MRI Mode on the RNS System programming tablet
Open Up New Possibilities for Patients
Who may require MRI, including patients with:
- Tuberous sclerosis
- Brain tumors
- Multiple sclerosis
- MRI needs outside of the brain
- Patients who may benefit from laser ablation
Neurology FAQs
RNS System MRI Conditional Labeling
What has changed with the updated MRI labeling for the RNS System?
Patients with the RNS® Neurostimulator (model RNS-320) can now receive a 1.5T (Tesla) Full Body MRI under certain conditions.
The full MRI Conditional conditions can be found in the MRI Guidelines for the RNS System.
What model of the RNS System is MRI conditional?
Only the RNS-320 model of the RNS System and NeuroPace leads are MRI Conditional. The RNS-300M is MRI Unsafe. Please see the MRI Guidelines for the RNS System for more details.
Is the RNS System full body MRI compatible?
A patient implanted with the RNS-320 Neurostimulator and NeuroPace leads can receive an MRI anywhere on the body. The RNS-320 Neurostimulator and NeuroPace leads are approved by FDA for full-body conditional MRI scans.
What strength MRI scan can my patients get with the RNS System?
The RNS System’s MR conditional labeling is for 1.5T (Tesla) MRI strength only.
Please see the MRI Guidelines for the RNS System for more details.
What is the process for ensuring that my patient can safely receive an MRI?
- Obtain a copy of the following forms (all found in the MRI Guidelines for the RNS System):
- Schedule patient appointment to turn MRI Mode ON as close as possible to MRI scan date
- Verify that all criteria are met for patient eligibility per the MRI Guidelines for the RNS System
- Interrogate the patient’s implanted RNS Neurostimulator (model RNS-320) and program MRI Mode ON
- Fill out and date the Neurology RNS System MRI Pre-Scan Checklist
- Send the RNS System MRI Pre-Scan Checklist and the Radiology RNS System MRI Scan Checklist forms with the patient to bring to their MRI
- Schedule a return appointment to have MRI Mode turned OFF as soon as possible after the MRI
Are the MRI scan conditions compatible with a laser ablation surgery?
With MRI Conditional labeling, the RNS Neurostimulator (model RNS-320) can be implanted prior to a laser ablation under certain conditions. Please check with the manufacturer of the laser to determine the conditions under which this procedure could be safe.
RNS® System MRI Mode
How do I turn MRI Mode ON for my patient?
- Interrogate the RNS Neurostimulator (model RNS-320 only)
- Click on the Neurostim Info tile
- Hold the wand within one inch of the neurostimulator and click Turn MRI Mode ON in the bottom right of the screen
- The programmer immediately sends the programming signal to turn MRI Mode ON and the screen shows when MRI Mode has been turned on
Why is it important to turn MRI Mode OFF as soon as possible after the MRI?
While in MRI Mode, the neurostimulator is not detecting or delivering therapy to the patient. Also, MRI Mode uses more battery than normal operating mode. It is important to turn MRI Mode OFF as soon as possible to restore detection and therapy, and end extra battery consumption.
How do I turn MRI Mode OFF for my patient?
If the device has MRI Mode ON:
- Interrogate the RNS Neurostimulator (model RNS-320 only)
- Click on the Neurostim Info tile
- Hold the wand within one inch of the neurostimulator and click Turn MRI Mode OFF in the bottom right of the screen
- The programmer immediately sends the programming signal to turn MRI Mode OFF and the screen shows when MRI Mode has been turned off
What would happen if a patient received an MRI without MRI Mode ON?
If MRI Mode is not turned ON, the RNS System may be damaged or malfunction, and result in serious patient risks. Only use conditions described in the MRI Guidelines for the RNS System, which includes information on patient safety.
What happens to the RNS System when MRI Mode is ON?
MRI Mode turns stimulation and detection off, but there are other processes that are still operating. Importantly, turning stimulation and detection off manually is not the same as placing the device into MRI Mode.
Can I see when MRI Mode is turned ON for my patient?
Yes, you can check on MRI Mode status at any time through the NeuroPace Patient Data Management System (PDMS).
Radiology FAQs
RNS System MRI Conditional Labeling
Is the RNS System MRI Conditional?
Patients with RNS Neurostimulator (model RNS-320) can now receive a 1.5T (Tesla) Full Body MRI under certain conditions. The full MRI Conditional conditions can be found in the MRI Guidelines for the RNS System.
Prior to conducting the MRI, review the Radiology RNS System MRI Scan Checklist to ensure all necessary conditions are met.
What model of the RNS System is MRI conditional?
Only the RNS-320 model of the RNS System and NeuroPace leads are MRI Conditional. The RNS-300M is MRI Unsafe. Please see the MRI Guidelines for the RNS System for more details.
Where can I find a copy of the conditions under which an MRI can be conducted with an RNS System?
Refer to the Radiology RNS System MRI Scan Checklist to ensure compliance with the pre-scan requirements and MR scan conditions in the MRI Guidelines for the RNS System. These guidelines detail the steps necessary for a patient implanted with an RNS Neurostimulator (model RNS-320) and NeuroPace leads to safely obtain an MRI.
What are the MRI system specifications necessary for an MRI on patients implanted with an RNS Neurostimulator (model RNS-320) and NeuroPace leads?
The MRI scanner must meet the following specifications:
- Horizontal field, closed-bore (cylindrical) system
- Static magnetic field strength of 1.5T (Tesla)
- Spatial field gradient ≤ 30 T/m (3,000 gauss/cm)
- Gradient slew rate ≤ 200 T/m/s per axis
Please refer to the MRI Guidelines for the RNS System for the full list of conditions for a safe MRI.
Are the RNS Neurostimulator (model RNS-320) and NeuroPace leads full body MRI compatible?
A patient implanted with the the RNS Neurostimulator (model RNS-320) and NeuroPace leads can receive an MRI anywhere on the body. The RNS Neurostimulator (model RNS-320) and NeuroPace Leads are approved by FDA for full-body conditional MRI scans.
Please refer to the MRI Guidelines for the RNS System for the full list of conditions for a safe MRI.
What is the process for conducting an MRI on a patient implanted with the RNS Neurostimulator (model RNS-320) and NeuroPace leads?
- Review the completed Neurology RNS System MRI Pre-Scan Checklist to confirm that all pre-scan requirements have been met, including that the patient has an eligible device and that MRI Mode has been turned ON.
- Prior to conducting the MRI, review the Radiology RNS System MRI Scan Checklist to ensure all necessary scan conditions are met.
- After the MRI is complete, remind the patient that it is important to return to their Epilepsy Specialist to have MRI Mode turned OFF because the RNS Neurostimulator is not delivering therapy and uses more battery while MRI Mode is ON.
- Please refer to the MRI Guidelines for the RNS System for the full list of MRI safety conditions.
Where can I find information on whether an RNS System patient is eligible for an MRI?
- You should receive a copy of the Neurology RNS System MRI Pre-Scan Checklist completed by the patient’s epilepsy specialist which indicates the RNS Neurostimulator model number and confirms the patient’s eligibility for an MRI.
- The RNS System Neurostimulator model number can also be found on the Patient Identification card. If there is a discrepancy between the model number information on the card and the checklist, confirm the model number with the patient’s epilepsy specialist before conducting the scan.
- Additionally, you can contact our customer service department at 1-866-726-3876 for more details.
RNS System MRI Scan Conditions
Is there a limit to the number of times a patient implanted with the RNS Neurostimulator (model RNS-320) and NeuroPace leads can be scanned?
There is not a limit to the number of times that a patient with the RNS System can be scanned.
Is there a time limit to how long a patient can receive an active scan?
There is a time limit per active scan. The active scan needs to be ≤ 30 minutes per imaging session. Wait 30 minutes between sessions.
What type of coils can be used with the RNS Neurostimulator (model RNS-320) and NeuroPace leads?
Compatible: Full body RF transmit receive coil (quadrature only), or full body RF transmit coil (quadrature only) with any receive only coil can be used with the RNS Neurostimulator (model RNS-320).
NOT compatible: Do not use a head or extremity transmit coil.
Please see the MRI Guidelines for the RNS System for more detail.
Are there guidelines on the appropriate B values and SAR values?
Yes, there are guidelines for both values in the labeling based on body scan region.
Please refer to the MRI Guidelines for the RNS System for the full list of MRI safety conditions.
Are there different Operating Mode requirements for different areas of the body?
Yes, there are three zones with different Operating Mode conditions.
Please refer to the MRI Guidelines for the RNS System for the full list of MRI safety conditions.
What would happen if a patient received an MRI without MRI Mode ON?
If MRI Mode is not turned ON, the RNS System may be damaged or malfunction, and result in serious patient risks. Only use conditions described in the MRI Guidelines for the RNS System, which includes information on patient safety.
*Please check with the manufacturer of the laser to determine the conditions under which this procedure could be safe.
Refer to the MRI Guidelines for the RNS System to determine patient eligibility for MRI and for the specific conditions required to safely perform an MRI scan on patients implanted with an RNS Neurostimulator model RNS-320. All NeuroPace manuals are available at www.NeuroPace.com or by contacting NeuroPace, Inc.


