RMN de RNS® System de NeuroPace: Una guía para pacientes con RNS-320
Pasos que seguir antes, durante y después de la RMN (resonancia magnética)
#1: Comuníquese con su especialista en epilepsia para hablar sobre sus necesidades de RMN
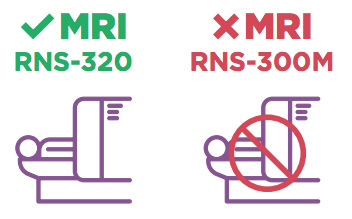
Si el médico le ha recomendado que se realice una RMN, comuníquese con su especialista en epilepsia para confirmar que tiene un RNS Neuroestimulador modelo RNS-320 y analice los próximos pasos.
Los pacientes con el RNS Neuroestimulador modelo RNS-300M aún NO PUEDEN realizarse una RMN. El modelo RNS-300M no es seguro para una RMN.
#2: Programe la RMN
Cuando programe una cita para la RMN, diga que tiene un RNS Neuroestimulador (modelo RNS-320) y cables implantados en la cabeza.
Lo mejor para usted será realizarse la RMN en el mismo centro donde se encuentra el especialista en epilepsia.
#3: Programe dos (2) citas con su especialista en epilepsia
Programe la primera cita con el especialista en epilepsia justo antes de la RMN para que pueda ACTIVAR el modo de resonancia magnética.
Programe la segunda cita para que se lleve a cabo inmediatamente después de la RMN para poder DESACTIVAR el modo de resonancia magnética.
Trate de planificar estas citas lo más cerca posible de la RMN, porque su Neuroestimulador RNS® no está administrando la terapia y usa más batería mientras el modo de resonancia magnética está ACTIVADO.
#4: Realizarse una RMN
Visite a su especialista en epilepsia justo antes de la RMN para ACTIVAR el modo de resonancia magnética. El especialista le dará una lista de verificación de RMN de RNS System para que la lleve a donde se le realizará el procedimiento.
Dígale al radiólogo o al técnico de RMN que tiene un Neuroestimulador RNS y cables implantados en la cabeza y entrégueles la lista de verificación de RMN de RNS System de su especialista en epilepsia.
- Lleve su tarjeta de identificación de paciente del Neuroestimulador RNS al lugar donde se le realizará la RMN.
- No lleve el imán ni la computadora portátil con monitor remoto al lugar donde se le realizará la RMN. El imán y la computadora portátil con monitor remoto no son seguros para la RMN y no son necesarios durante el procedimiento.

#5: Después de la RMN
Tan pronto como sea posible después de la RMN (resonancia magnética), visite al especialista en epilepsia para que pueda DESACTIVAR el modo de resonancia magnética. Lleve las listas de verificación de RMN de RNS System.
Preguntas frecuentes
¿Es seguro para mí realizarme una RMN con RNS System?
La Administración de Alimentos y Medicamentos (FDA) ha aprobado el uso de RMN (resonancia magnética) para pacientes con el Neuroestimulador modelo RNS-320 y cables NeuroPace bajo ciertas condiciones de RMN. Los pacientes con el Neuroestimulador modelo RNS-300M aún NO PUEDEN realizarse una RMN. Comuníquese con el especialista en epilepsia que administra su sistema de RNS para discutir la posibilidad de realizarse una resonancia magnética y consulte el Manual del paciente de RNS System para obtener información acerca de realizarse una RMN con RNS System.
¿Cuáles son las condiciones para realizarme una RMN si tengo colocado El sistema RNS?
Es importante tener en cuenta que debe tener RNS System Neuroestimulador modelo RNS-320 para realizarse una RMN. Los pacientes con el Neuroestimulador modelo RNS-300M aún NO PUEDEN realizarse una RMN. Para pacientes con el modelo RNS-320, el especialista en epilepsia que administra RNS System debe programarlo en modo de resonancia magnética para garantizar la seguridad durante una RMN. Hable con su especialista en epilepsia y consulte el Manual del paciente de RNS System para obtener información acerca de realizarse una RMN con sistema RNS.
¿En qué partes del cuerpo puedo realizarme una RMN si tengo sistema RNS?
Puede realizarse una RMN en cualquier parte del cuerpo. El Neuroestimulador RNS-320 y los cables NeuroPace están aprobados por la FDA para RMN de cuerpo entero condicionales.
¿Debo ir un centro especial para realizarme una RMN?
Lo mejor es que vaya al centro médico donde se atiende con el especialista en epilepsia. Deberá ir a un centro que tenga una máquina para RMN de 1.5T y que también tenga acceso a un programador de sistema RNS. Haga clic aquí para encontrar un centro RNS cerca de usted.
¿Dónde puedo encontrar información sobre cómo realizarme una RMN con sistema RNS?
Comuníquese con el especialista en epilepsia que administra su RNS System para discutir la posibilidad de realizarse una resonancia magnética y consulte el Manual del paciente de RNS System para obtener información acerca de realizarse una RMN con Sistema RNS.
¿Qué modelo de Sistema RNS es compatible con las RMN?
Solo el RNS System Neuroestimulador modelo RNS-320 es elegible para resonancias magnéticas bajo ciertas condiciones. Las RMN NO PUEDEN realizarse con el Sistema RNS Neuroestimulador modelo RNS-300M.
El número de modelo de su Sistema RNS figura en la tarjeta de identificación de paciente. También puede comunicarse con su especialista en epilepsia o con el equipo de servicio al cliente de NeuroPace (1-866-726-3876) para averiguar qué modelo el Sistema RNS System le han implantado.
¿Cómo puedo saber qué modelo de RNS System tengo?
El número de modelo de su Sistema RNS System figura en la tarjeta de identificación de implante médico. También puede comunicarse con su especialista en epilepsia o con el equipo de servicio al cliente de NeuroPace (1-866-726-3876) para averiguar qué modelo el Sistema RNS le han implantado.
¿Qué sucede si me explantan el Sistema RNS o algunas de sus piezas? ¿Aún soy elegible para una RMN?
Comuníquese con su especialista en epilepsia para determinar si las piezas restantes son compatibles con las RMN.
¿Debo llevar mi imán y mi computadora portátil con monitor remoto al lugar donde se realizará la RMN?
No. No lleve el imán ni la computadora portátil con monitor remoto al lugar donde se le realizará la RMN. No es seguro llevar estos elementos al lugar donde se realiza la RMN.
¿Qué es el modo de resonancia magnética y por qué es importante DESACTIVARLO cuanto antes después de la RMN?
Su especialista en epilepsia debe poner el Neuroestimulador en modo de resonancia magnética antes de realizarse la RMN para su seguridad y para proteger el Neuroestimulador de daños. Mientras está en modo de resonancia magnética, el Neuroestimulador no administra la terapia y usa más batería que en el modo normal. Después de realizarse la RMN, es importante DESACTIVAR el modo de resonancia magnética lo antes posible para restablecer la terapia y volver al uso normal de la batería.
Para pacientes con RNS-320: Solicite una nueva tarjeta de identificación de implante médico
Consulte las Pautas para RMN para el Sistema RNS System para determinar la elegibilidad del paciente para una RMN y las condiciones específicas requeridas para realizar de manera segura una RMN en pacientes implantados con un RNS Neuroestimulador modelo RNS-320. Todos los manuales de NeuroPace están disponibles en www.NeuroPace.com o comunicándose con NeuroPace, Inc.


