

Clinical Study
For teens aged 12 through 17 years

If medications are not providing seizure control, the RESPONSE Study may be the right next step.
This research study is evaluating the safety and effectiveness of the NeuroPace RNS® System as a treatment option for drug-resistant, focal epilepsy in patients aged 12 through 17 years.
CAUTION—Investigational device. Limited by United States law to investigational use for the following Indications for Use:
The RNS System investigational intended use is as an adjunctive therapy in reducing the frequency of seizures in individuals 12 through 17 years of age with partial onset seizures who have undergone diagnostic testing that localized no more than 2 epileptogenic foci, are refractory to two or more antiepileptic medications, and currently have frequent and disabling seizures (motor partial seizures, complex partial seizures and/or secondarily generalized seizures).
Find out if your teenager could be eligible for the  Study.
Study.
Children’s Hospital of Orange County (CHOC)
Orange, CA 92868
Study Coordinator: Angelyque Lorenzana
Email: Angelyque.Lorenzana@choc.org
Phone: 714-509-8972. ext.18972
Nicklaus Children’s Hospital
Miami, FL 33155
Study Coordinator: Mari Vega
Email: Marinellie.Vega@Nicklaushealth.org
Phone: 786-624-3516
University of Chicago
Chicago, IL 60637
Study Coordinator: Christine Herrera
Email: crodriguez6@bsd.uchicago.edu
Phone: 773-702-2256
Spectrum Health
Grand Rapids, MI 49503
Study Coordinator: Shannon Emrich
Email: Shannon.Emrich@spectrumhealth.org
Phone: 616-391-0174
Mayo Clinic Minnesota
Rochester, MN 55905
Study Coordinator: Bridget Neja
Email: Neja.Bridget@mayo.edu
Phone: 507-538-0266
New York Medical College/Westchester Medical Center
Hawthorne, NY 10532
Study Coordinator: Donika Zogejani
Email: dzogejan@nymc.edu
Phone: 914-768-3970
Cohen’s Children’s Medical Center
Lake Success, NY 11042
Study Coordinator: Aaqil Ali
Email: aali27@northwell.edu
Phone: 631-290-1193
Mount Sinai Beth Israel
New York, NY 10029
Study Coordinator: Armand Harb
Email: Armand.Harb@mountsinai.org
Phone: 212-241-2524
LeBonheur Children’s Hospital
Memphis, TN 38103
Study Coordinator: Nick Leal
Email: Nicolas.Leal@lebonheur.org
Phone: 901-287-5886
What is Drug-Resistant
Epilepsy?
If you have tried at least two anti-seizure medications and still experience seizures, you may have drug-resistant epilepsy. Doctors may also refer to this type of epilepsy as refractory, intractable, hard-to-treat, or uncontrolled seizures. Over 1 million people in the U.S. are living with drug-resistant epilepsy.
What is the RNS System?
The RNS System is a medical device designed to treat drug-resistant focal epilepsy. The RNS System consists of an implantable neurostimulator and small wires (called leads). The leads are placed on and/or inside the brain where seizures start. Once placed, the device is designed to be unnoticed by others.

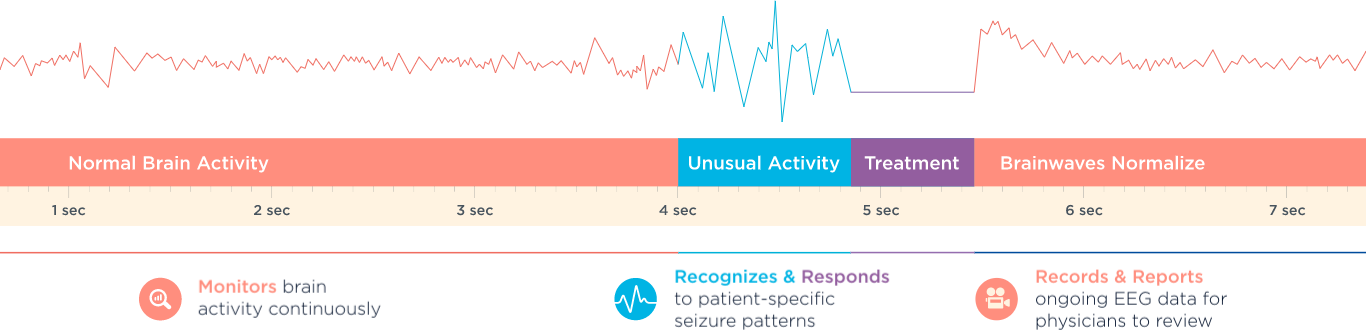
How Does the RNS System Work?
- It monitors brain activity continuously
- It is programmed to recognize and respond to your brain patterns, sending brief stimulation to help prevent seizures before they start.
- It records and reports your EEG information for your doctor to review.

FAQs
Why is this study being done?
The purpose of the RESPONSE Study is to demonstrate whether the NeuroPace RNS System is safe and effective (works well) as an additional treatment in patients implanted at ages 12 through 17 who have drug-resistant focal epilepsy
How long will I be in this study?
You will be in the RESPONSE Study for 2 years after you are implanted with the RNS System.
What are my responsibilities if I take part in this study?
If you take part in this study you will be asked to do the following:
- Enter information in an electronic diary every day.
- Attend all study visits (~3-4 times a year, in-person or telehealth) and complete all study activities.
- Tell the study team about any side effects, illnesses and injuries (including hospitalizations) you have while you are in the study.
- Use the NeuroPace Remote Monitor to send information to your doctor.
